UK Regulator Approves a New Skin Test for TB
7 November 2025
A newly approved diagnostic test could give UK clinicians a more accurate way to identify latent and active tuberculosis (TB).
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved Siiltibcy, a next-generation skin test that uses recombinant proteins to identify Mycobacterium tuberculosis infection with higher specificity than current methods.
The approval authorises Serum Life Sciences Ltd to market the test for use in both adults and children. It follows an earlier recommendation for marketing authorization by the European Medicines Agency in October 2024.
TB remains one of the world’s leading infectious diseases, affecting more than 10 million people globally each year. Unlike traditional purified protein derivative (PPD) tests, Siiltibcy’s recombinant design reduces false positives caused by prior Bacille Calmette-Guérin vaccination, potentially improving diagnostic accuracy in low-incidence countries such as the UK and across Europe.
How the Test Works
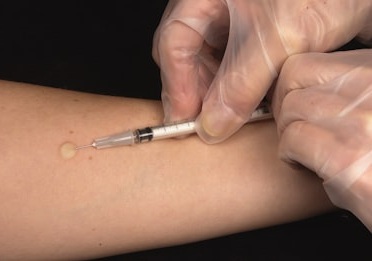
Siiltibcy is administered via intradermal injection on the forearm. It contains two synthetic antigens, rdESAT-6 and rCFP-10, which are highly specific to M tuberculosis. The test detects a T-cell-mediated immune response.
In previously infected individuals, the immune system responds to these proteins by releasing inflammatory cytokines, causing induration (hardening) at the injection site. The reaction is typically assessed 48-72 hours post-injection by measuring the induration size to determine the presence of M tuberculosis infection or active disease.
Clinical Evidence
Three main studies involving 2625 individuals, including children, compared Siiltibcy with two authorised diagnostic tests for identifying M tuberculosis: PPD, which uses the same skin injection technique as Siiltibcy; and QuantiFERON-TB Gold in-Tube (QFT), a blood-based test.
Participants included individuals unexposed to M tuberculosis, those with suspected infection, and confirmed TB cases. Results showed that Siiltibcy’s positivity rate increased with exposure, demonstrating sensitivity between 68% and 79% — comparable with QFT and slightly lower than PPD.
Specificity ranged from 83% to 97%, slightly higher than either PPD or QFT.
Safety Profile and Contraindications
The most common side effects include pruritus (itching) at the injection site, which may affect about 1 in 5 people, and bruising or pain at the site, which may affect up to 1 in 10.
Siiltibcy must not be used in people hypersensitive to Lactococcus lactis — used in its production — or to its active substances or other ingredients. It should also be avoided in those who have had severe local or general reactions to other TB skin tests.
Full safety information is available in the Patient Information Leaflet and Summary of Product Characteristics, which will be published on the MHRA website within seven days of approval.
By Zeel Mehta
Source: Medscape